Doppler shift for de-broglie’s matter waves
Derivation of de Broglie Wavelength 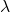
Start with the total energy of a de Broglie matter wave, combining rest mass and kinetic energy:
![]()
Multiply both sides by ![]() to eliminate denominators:
to eliminate denominators:
![]()
Rewriting in standard quadratic form:
![]()
This is a quadratic equation in ![]() , and we solve it using the quadratic formula:
, and we solve it using the quadratic formula:
![Rendered by QuickLaTeX.com \[ \lambda = \frac{-(-h v) \pm \sqrt{(-h v)^2 - 4 \cdot mc^2 \cdot \frac{h^2}{2m}}}{2 mc^2} \]](https://stationarystates.com/wp-content/ql-cache/quicklatex.com-e54bcdb7346d7628293e192fce6bd712_l3.png)
Simplifying:
![]()
Or, factoring out constants:
![]()
We choose the positive root to ensure that ![]() :
:
![]()
Now, we have the wavelength in terms of the wave velocity. We can calculate the doppler shift.
![]()
Factorizing out common terms:
(★) ![]()
To calculate the Doppler shift, we find the differential change in wavelength for a small change in velocity ![]() :
:
![]()
Then the change in wavelength ![]() is approximately:
is approximately:
![]()
Final Result — Doppler Shift of de Broglie Wavelength:
![Rendered by QuickLaTeX.com \[ \boxed{ \Delta \lambda = \frac{h}{2mc^2} \left( 1 + \frac{v}{\sqrt{v^2 - 2c^2}} \right) \Delta v } \]](https://stationarystates.com/wp-content/ql-cache/quicklatex.com-32d251f56127949791b962c6d8abacfe_l3.png)