Spin in Bohmian Quantum Mechanics
Electron Spin in Bohmian Mechanics
Does Bohmian mechanics predict electron spin?
No, Bohmian mechanics does not independently predict the existence of spin.
Instead, it reproduces the predictions of standard quantum mechanics by incorporating spin into the wave function.
1. Original Bohm Model (1952)
- Treated spinless particles.
- Particles have definite positions, guided by the wavefunction.
- Wavefunction evolves via the Schrödinger equation.
2. Inclusion of Spin
- Wavefunction becomes a two-component spinor:
ψ = [ψ+, ψ-] - Evolves under the Pauli equation.
- Guiding equation still only involves position.
3. What About Spin Itself?
- No hidden variable for spin in standard Bohmian mechanics.
- Spin affects motion through the wavefunction’s internal structure.
- Spin measurement outcomes result from:
- Definite particle position
- Wavefunction structure under external fields
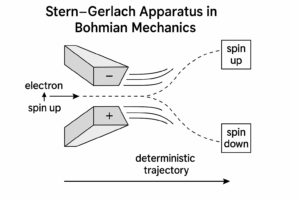
Experimental Predictions
Bohmian mechanics gives the same predictions as standard quantum theory for spin experiments (e.g., Stern–Gerlach), but explains them deterministically.
Summary
Bohmian mechanics assumes spin as part of the wavefunction structure, and explains spin measurement outcomes via deterministic particle motion — but it does not derive spin from deeper first principles.