E=hv – careful when applying to material particles
DeBroglie taught us that not just photons, but all particles have an energy proportional to their wave like characteristic.
For electrons and other material particles, this begs the question – what is E in the equation below?
![]()
- Is E equal to the rest energy
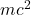 ?
? - Or is it equal to the rest energy plus the binding energy (electron in an atom)?
- Or it is equal to rest energy plus binding energy plus it’s kinetic energy?
Actually, it could be any of the three above. Hence, one must be careful what one means by E=h\nu when applied to electrons and other mass bearing particles.
The Resolution?
The actual E in the equation above is not important. What’s important are the Energy differences. When the electron jumps from an allowed state to another allowed state, it emits photons of energy equal to E. And that is exactly what the E in the ![]() represents for an electron.
represents for an electron.
The Fundamental disagreement between Relativity and QM
I spent a lot of time studying this paper on the Fundamental Disagreement of Wave Mechanics with Relativity
These kind of paradoxes can be resolved when one keeps in mind that the E above is not really the energy of the material particle, but rather, the energy emitted when the particle changes between states. The paper above is still legit – and highlights a slightly different issue.